Products
JBA Health is focused on providing new approaches for healthcare worldwide.
- Wearable electrical nerve stimulation devices - to manage the pain associated with opioid withdrawal.
- Regenerative medicine - to repair or grow human tissue and organs in the laboratory with cell-based technologies.
- • AI assisted healthcare software - to bring about transformative change for hospitals, physicians, and patients.

Emma Stone
General Practitioner
“Fusce vitae commodo ipsum, eu ullam corper magna nam non posuere.”

Years
Already Experienced In The World of Health

Introducing the
Sparrow Ascent™ device
Non-Addictive
Drug-Free
FDA Cleared
Prescription Required
Safe
Comfortable (no needles)
Note: Spark Biomedical, Inc. is the manufacturer of the Sparrow Ascent device. Please inquire about the availability of the Sparrow Ascent device in your geographic region.
Physician Testimonials
Michael Sprintz, DO, FMASAM
The Sparrow Therapy System expands my options for patients who are experiencing opioid withdrawal, regardless of whether they have OUD or are just physically dependent and need to taper down or discontinue opioids altogether. I'm no longer stuck with just MAT or Abstinence. Finally, I have another option that gets my patients safely and effectively through opioid withdrawal without risks of overdose or diversion while also giving them the relief they need to stay in treatment and build a strong foundation in recovery.
Triple-board-certified expert in addiction and chronic pain, Sprintz Center for Recovery/ Sprintz Center for Pain
The Data
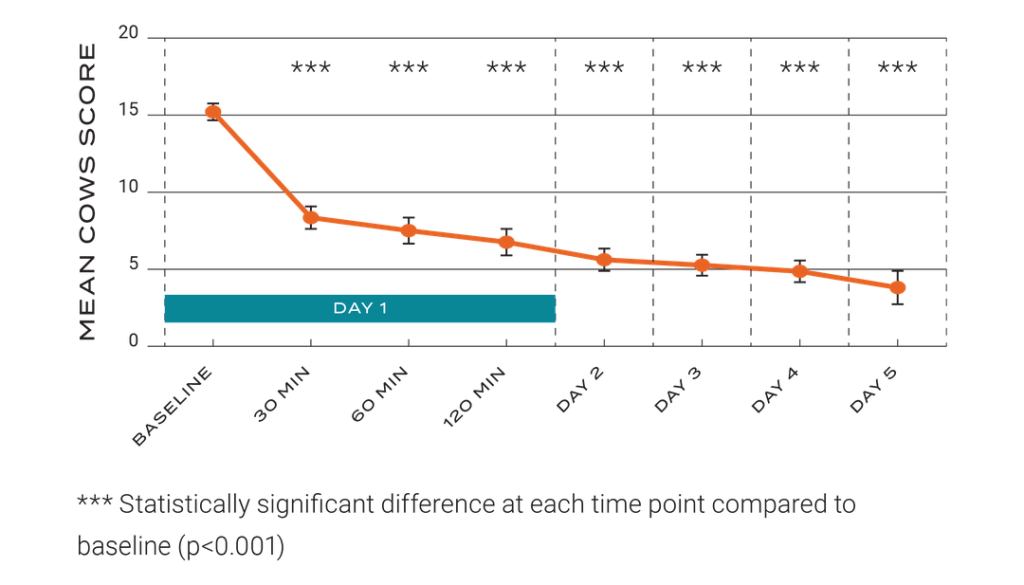
Sparrow Ascent is the first and only FDA-cleared, non-invasive wearable neurostimulation device for opioid withdrawal relief backed by a Level 1 clinical trial. More importantly, our studies have shown Sparrow Ascent begins to provide significant opioid withdrawal symptom relief in as little as 30 minutes.
Here’s what we discovered in the 31 patients suffering from opioid withdrawal symptoms with a Clinical Opiate Withdrawal Scale (COWS) score >15:
84%of participants experienced mild or no withdrawal symptoms after 60 minutes of therapy.

100% of participants sustained a clinically meaningful reduction in withdrawal symptoms by day 3.

9 out of 10 participants who completed detox accepted a referral to continue opioid use disorder treatment.
*15% or greater reduction in COWS is considered clinically meaningful.
Source: Research and data provided by Spark Biomedical, Inc. the manufacturer of the Sparrow Ascent device.
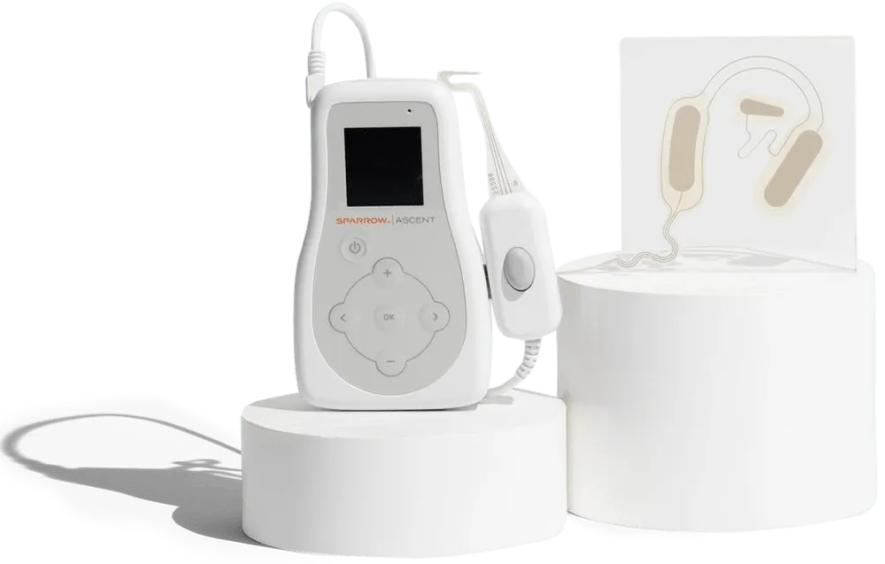
Sparrow Ascent Kit
Sparrow Ascent Includes
Disposable Earpieces
Sparrow Ascent is the first and only FDA-cleared, non-invasive wearable neurostimulation device for opioid withdrawal relief backed by a Level 1 clinical trial.
More importantly, our studies have shown Sparrow Ascent begins to provide significant opioid withdrawal symptom relief in as little as 30 minutes. Here’s what we discovered in the 31 patients suffering from opioid withdrawal symptoms with a Clinical Opiate Withdrawal Scale (COWS) score >15:
Cable
It connects and delivers electrical impulses from the Patient Controller to the Earpiece.
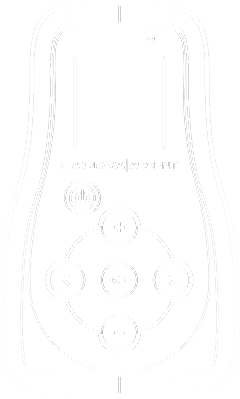
Patient Controller
It powers the Earpiece and puts you in control of the amount of relief you need. Color LCD screen provides at-a-glance information on your therapy.
